Partner with IQzyme for your Medical Device Manufacturing License needs and benefit from our expertise in navigating CDSCO regulations. Our comprehensive services are designed to streamline the licensing process, ensuring your products are compliant, safe, and ready for market.

Medical Device Manufacturing License: Seamless Solutions with IQzyme
At IQzyme, we specialise in providing expert services for obtaining a Medical Device Manufacturing License, ensuring that your products meet the stringent requirements set by the Central Drugs Standard Control Organization (CDSCO). Our comprehensive approach covers every step of the licensing process, making it easier for you to navigate the complexities of regulatory compliance. We start by thoroughly understanding the specific regulatory guidelines that apply to your medical devices, including the necessary documentation, classification, and testing requirements as outlined by the CDSCO.
Our team of experienced professionals works closely with you to develop a tailored strategy that addresses all regulatory aspects. This includes preparing and submitting the necessary documentation, such as the Device Master File (DMF) and Site Master File (SMF), which are crucial for demonstrating compliance with CDSCO standards. We also assist in the implementation and maintenance of a robust Quality Management System (QMS) that aligns with CDSCO regulations, ensuring that all manufacturing processes are controlled and documented according to the highest quality standards.
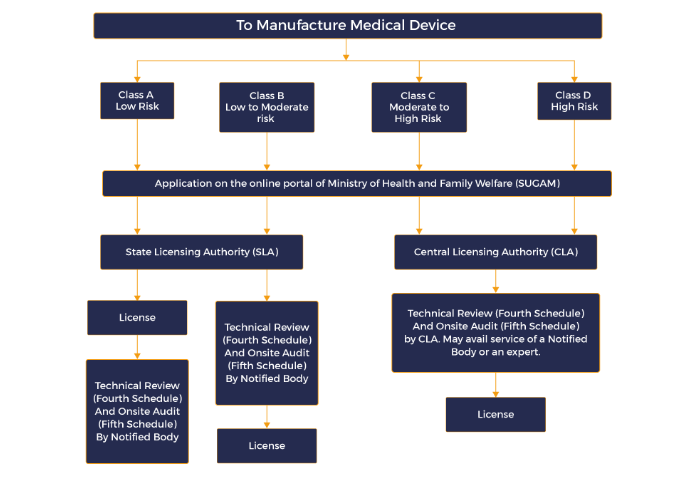
Steps in CDSCO licensing:
Identify the risk class of medical device-: The CDSCO categorises devices based on potential risk (Class A, B, C, or D). All notified Class A, B, C and D devices necessitate a manufacturing licence.
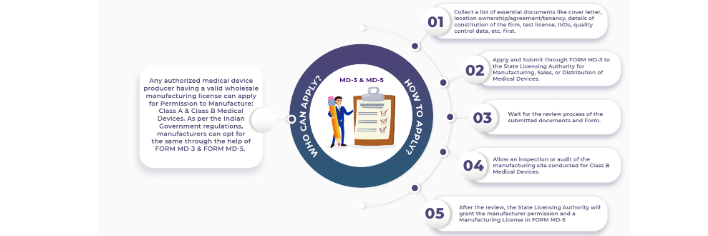
For Class A and B medical device application submitted in form MD 3 along with required technical documents and after audit process SLA (State licensing authority) issue licence in MD 5
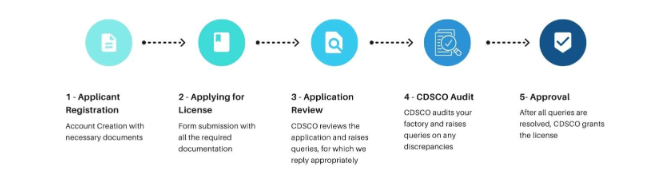
For Class C and D medical device application submitted in form MD 7 along with required technical documents and after audit process CLA (Central licensing authority) issue licence in MD 9
Licence renewal is necessary before it expires.
For a newly developed medical device manufacturer need to apply test licence in MD 12 and obtain licence in MD 13

Quality Management System (QMS): Your Path to CDSCO Compliance with IQzyme
A robust Quality Management System (QMS) is essential for securing your Medical Device Manufacturing License and ensuring long-term success in the highly regulated medical device industry. At IQzyme, we specialise in helping you develop and implement a QMS that is fully aligned with the stringent standards set by the Central Drugs Standard Control Organization (CDSCO). Our comprehensive QMS services are designed to meet CDSCO requirements while also enhancing your operational efficiency and product quality.
Our team of experts begins by conducting a thorough assessment of your current processes and identifying areas for improvement. We then assist you in establishing effective processes and creating detailed, CDSCO-compliant documentation that covers every aspect of your operations, from design and development to production and post-market surveillance. This documentation includes standard operating procedures (SOPs), quality manuals, and work instructions, all tailored to meet CDSCO guidelines.
At IQzyme, we understand that a QMS is not a one-time implementation but a dynamic system that requires continuous improvement to adapt to evolving regulatory expectations and market demands. Our experts provide ongoing support to ensure your QMS remains up-to-date and effective. This includes regular audits, risk assessments, and training programs to keep your team informed about the latest CDSCO regulations and best practices.
By partnering with IQzyme, you benefit from our deep expertise in CDSCO standards and our commitment to quality and compliance. We help you build a culture of quality within your organisation, ensuring that every product you manufacture meets the highest standards of safety and efficacy. With a well-implemented QMS, you can confidently navigate the complexities of CDSCO compliance, reduce the risk of regulatory issues, and enhance your reputation in the medical device market.
Choose IQzyme for your QMS needs and ensure your medical devices not only meet but exceed CDSCO requirements. Our tailored solutions and expert guidance provide you with the assurance and peace of mind to focus on innovation and growth, knowing that your quality management processes are in expert hands.
Risk Analysis
Effective risk analysis is a cornerstone in the medical device industry, ensuring the safety and efficacy of products throughout their lifecycle. At IQzyme, our comprehensive risk analysis services are designed to help you proactively identify, evaluate, and mitigate potential risks associated with your medical devices. We employ industry-standard methodologies such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) to systematically assess all aspects of your device's design, production, and usage. This rigorous approach allows us to pinpoint potential failure modes and hazards, assess their potential impact, and prioritise mitigation strategies.
Our experts work closely with your team to develop a thorough risk management plan that not only meets but exceeds CDSCO requirements. We provide detailed documentation and analysis, helping you implement effective control measures and monitor their effectiveness over time. By integrating risk analysis into every stage of your product development and post-market surveillance, IQzyme ensures that your devices maintain high standards of safety and reliability, ultimately protecting patients and enhancing your product's market reputation. With our guidance, you can confidently navigate the complex regulatory landscape and demonstrate a strong commitment to quality and patient safety.
Medical Device Testing
At IQzyme, we offer expert assistance to guide you through the complexities of medical device testing, ensuring your products meet the stringent requirements set by the Central Drugs Standard Control Organization (CDSCO). While we do not perform the testing ourselves, our team provides invaluable support throughout the process. We help you identify the appropriate testing protocols, select accredited testing laboratories, and ensure that your test plans align with CDSCO regulations. Our expertise in navigating regulatory requirements ensures that your testing procedures are comprehensive, compliant, and tailored to meet the specific needs of your medical device. With IQzyme’s assistance, you can confidently manage your medical device testing requirements, facilitating a smoother path to achieving CDSCO approval and market readiness.
Clinical Evaluations and Repairs
Clinical evaluations are essential for validating the safety and performance of your medical devices. IQzyme supports you through the clinical evaluation process, providing guidance on study design, data analysis, and regulatory submission. Additionally, we offer repair services to address any issues identified during evaluations, ensuring your devices remain compliant with CDSCO standards.
Device Classification
Understanding the classification of your medical device is critical for obtaining the appropriate licence. IQzyme helps you navigate the CDSCO device classification system, ensuring that your product is classified correctly and meets all relevant regulatory requirements.
Documents Required For Manufacturing Licence:
- Constitution details
- Site ownership details
- ISO 13485 certificate
- DMF
- PMF
- Test licence certificate
- QMS documents
- Fee challan
- Legal form
By obtaining a CDSCO Manufacturing License, you demonstrate your commitment to patient safety and gain access to the vast Indian medical device market.
FAQ
Partner with IQzyme for your Medical Device Manufacturing License needs and benefit from our expertise in navigating CDSCO regulations. Our comprehensive services are designed to streamline the licensing process, ensuring your products are compliant, safe, and ready for market.

