
INDIAN MEDICAL DEVICE RULE 2017
The Indian Medical Device Rules of 2017 represent a comprehensive regulatory framework aimed at ensuring the safety, effectiveness, and quality of medical devices and in-vitro diagnostic devices in India. Enforced by the Central Drugs Standard Control Organization (CDSCO), these rules replace the Medical Device Rules of 1945 and align Indian regulations with international standards. The rules categorise medical devices into four classes—Class A, B, C, and D—based on their risk levels, with Class A being the lowest risk and Class D the highest. This classification helps in determining the level of scrutiny required for regulatory approval. For each class, specific requirements for medical device testing, manufacturing, labelling, and testing are prescribed to ensure adherence to high standards.
Manufacturers and importers of medical devices must obtain licences from CDSCO to ensure compliance with these regulations. The rules mandate detailed documentation and adherence to Good Manufacturing Practices (GMP) to guarantee product quality. Medical device manufacturing under these rules requires rigorous adherence to these GMP standards, ensuring that every device produced meets stringent quality benchmarks. The import of medical devices requires a specific licence, and devices must meet certain quality standards before being marketed in India. Additionally, the rules emphasise post-market surveillance, requiring manufacturers to monitor and report any adverse events or product failures, thus ensuring continuous oversight even after a device is approved for use.
For clinical trials involving medical devices, the rules stipulate that they must be conducted following strict protocols to ensure patient safety and the validity of trial results. Furthermore, there is a provision for the registration of clinical trial sites and ethics committees to uphold the integrity of the research process. The rules also establish a system for the fast-tracking of approvals for innovative devices that offer significant benefits over existing options, thereby encouraging the development of advanced medical technologies. This aspect ties closely with regulatory consulting services and compliance consulting, where IQZYME provides expertise in navigating the complexities of CDSCO compliance and ensuring that all regulatory submissions are meticulously prepared and timely filed.
The Indian Medical Device Rules of 2017 introduce a transparent and accountable system for regulatory compliance, which includes clear guidelines for product registration, quality assurance, and post-market monitoring. This regulatory framework aims to protect public health while facilitating the availability of high-quality medical devices in the Indian market. The focus on quality management systems (QMS) and risk management for medical devices ensures that manufacturers implement robust processes to identify, assess, and mitigate risks associated with their products. Through these rules, the Indian government seeks to harmonise its regulations with global standards, thereby fostering a safer and more efficient healthcare environment.
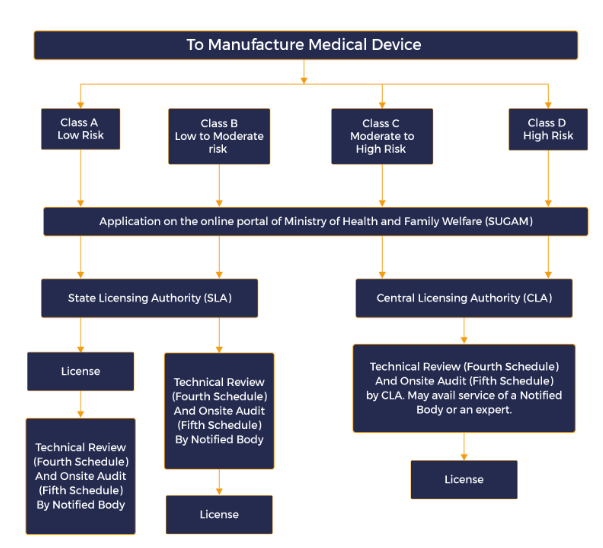
Medical device standards under these rules are aligned with international norms, ensuring that products meet global benchmarks for safety and performance. This alignment not only facilitates smoother entry of Indian-made devices into international markets but also ensures that devices imported into India meet stringent quality requirements. Regulatory affairs professionals play a critical role in this ecosystem, ensuring that all regulatory requirements are met throughout the product life cycle. The meticulous documentation and adherence to regulatory norms help in building a robust framework for medical device compliance, reducing the risk of product recalls and ensuring patient safety.
Regulatory submissions under the Indian Medical Device Rules involve comprehensive documentation that includes detailed product information, clinical data, and evidence of compliance with applicable standards. This thorough process is designed to ensure that only safe and effective devices are approved for use in the Indian market. Here at IQZYME, we provide valuable support in preparing these submissions, ensuring that all regulatory requirements are met and facilitating a smoother approval process.
In summary, the Indian Medical Device Rules of 2017 establish a stringent yet facilitative regulatory environment for medical devices in India. By emphasising medical device testing, manufacturing standards, post-market surveillance, and comprehensive risk management practices, these rules ensure that high-quality and safe medical devices are available to the Indian public. The involvement of regulatory consulting services and compliance consulting firms like IQZYME further enhances the industry's ability to navigate these regulations effectively, ensuring continuous CDSCO compliance and fostering innovation in the medical device sector. This regulatory framework not only protects public health but also aligns with global standards, making Indian medical devices competitive on the international stage.
For more information on how IQZYME can assist you with CDSCO projects and ensure compliance with the Indian Medical Device Rules of 2017, visit our website or contact our team of experts. We provide comprehensive regulatory consulting services, from regulatory submissions to quality management systems, helping you achieve and maintain compliance in a dynamic regulatory landscape.

